Transmembrane domains of fusion proteins promote stalk formation by inducing membrane disorder
Abstract
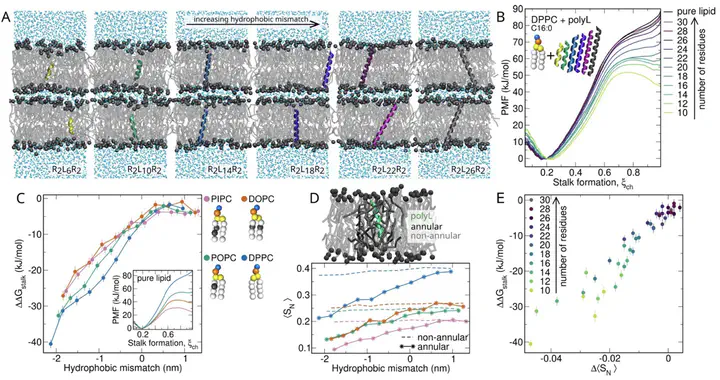
Membrane fusion is a fundamental process involved in exocytosis, fertilization, or cell entry by enveloped viruses. Membrane fusion is facilitated by fusion proteins, which are anchored in membranes by helical transmembrane domains (TMDs). Previous studies showed that TMD variations may alter the fusion efficiency, suggesting that TMDs are not merely passive anchors, however the mechanism by which TMDs drive fusion is not well understood. We used high-throughput coarse-grained molecular dynamics simulations and free energy calculations to quantify effects of TMDs on the formation of the first fusion intermediate, that is, of a fusion stalk. We analyzed five physiologically relevant TMDs derived from viral fusion proteins and the SNARE complex embedded in various lipid environments. We find that the addition of TMDs favors stalk formation by typically 10 to 30 kJ/mol in a concentration-dependent manner. Using helices with sequences R2LnR2 (n=6,…, 26), we find that negative hydrophobic mismatch between the TMD and the membrane core strongly promotes fusion. Analysis of the lipid tail order parameters of annular lipids revealed a strong correlation between stalk stabilization and induced lipid disorder. Together, our findings suggest that TMDs actively contribute to membrane fusogenicity by locally perturbing the membrane order.