Depletion of the protein hydration shell with increasing temperature observed by small-angle X-ray scattering and molecular simulations
Abstract
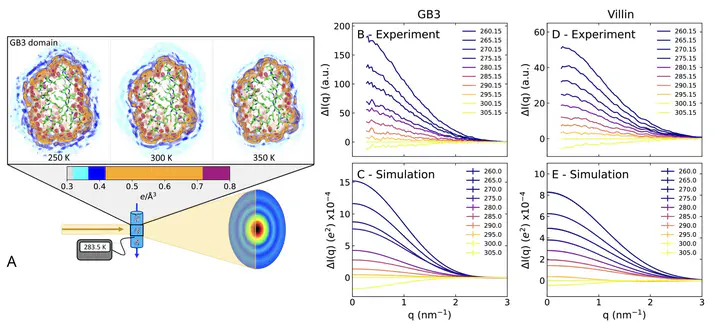
The hydration shell is an integral part of proteins since it plays key roles for conformational transitions, molecular recognition, and enzymatic activity. While the dynamics of the hydration shell have been described by spectroscopic techniques, the structure of the hydration shell remain less understood due to the lack of hydration shell-sensitive structural probes with high spatial resolution. We combined temperature-ramp small-angle X-ray scattering (T-ramp SAXS) from 255–335 K with molecular simulations to show that the hydration shells of the GB3 domain and villin headpiece are remarkably temperature-sensitive. For proteins in the folded state, T-ramp SAXS data and explicit-solvent SAXS predictions consistently demonstrate decays of protein contrasts and radii of gyration with increasing temperature, which are shown to reflect predominantly temperature-sensitive depleting hydration shells. The depletion is not merely caused by enhanced disorder within the hydration shells but also by partial displacements of surface-coordinated water molecules. Together, T-ramp SAXS and explicit-solvent SAXS calculations provide a novel structural view on the protein hydration shell, which underlies temperature-dependent processes such as cold denaturation, thermophoresis, or biomolecular phase separation.