Integrated structural dynamics uncover new modes of $\mathrm{B}_{12}$ photoreceptor activation
Abstract
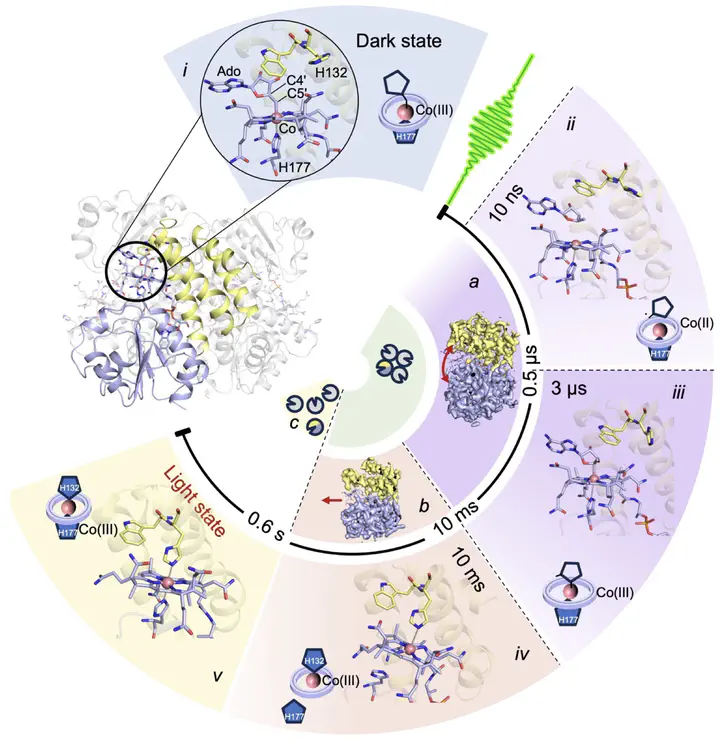
Photoreceptor proteins initiate, regulate and control fundamental biological processes such as vision, photosynthesis and circadian rhythms. A large photoreceptor subfamily uses vitamin B12 derivatives for light sensing, contrasting with the well-established mode of action of these organometallic derivatives in thermally activated enzymatic reactions. The molecular mechanism of B12 photoreception and how this differs to the thermal pathways remain unknown. Here we provide a detailed spatio-temporal description of photoactivation in the prototypical tetrameric B12 photoreceptor CarH from nanoseconds to seconds by using an integrative approach, combining time- and temperature-resolved structural and spectroscopic methods with quantum chemical calculations. High resolution structural snapshots of key intermediates illustrate how photocleavage of a Co–C bond triggers a pathway of structural changes that propagate throughout CarH from the B12 chromophore, via a previously unknown adduct, to finally cause tetramer dissociation. These unique intermediates, which differentiate CarH from thermally-activated B12 enzymes, steer the photoactivation pathway and act as the molecular bridge between photochemical and photobiological timescales. Our results offer a spatio-temporal understanding of CarH photoactivation and pave the way for designing and optimising B12-dependent photoreceptors for future optogenetic applications.