How arginine derivatives alter the stability of lipid membranes: dissecting the roles of side chains, backbone and termini
Abstract
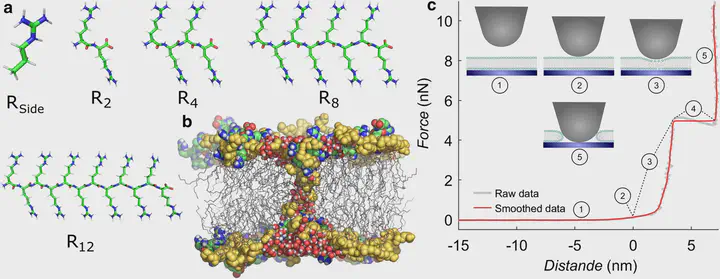
Arginine (R)-rich peptides constitute the most relevant class of cell-penetrating peptides and other membrane-active peptides that can translocate across the cell membrane or generate defects in lipid bilayers such as water-filled pores. The mode of action of R-rich peptides remains a topic of controversy, mainly because a quantitative and energetic understanding of arginine effects on membrane stability is lacking. Here, we explore the ability of several oligo-arginines $R_n$ and of an arginine side chain mimic $R_{Side}$ to induce pore formation in lipid bilayers employing MD simulations, free-energy calculations, breakthrough force spectroscopy and leakage assays. Our experiments reveal that $R_{Side}$ but not $R_n$ reduces the line tension of a membrane with anionic lipids. While $R_n$ peptides form a layer on top of a partly negatively charged lipid bilayer, $R_{Side}$ leads to its disintegration. Complementary, our simulations show $R_{Side}$ causes membrane thinning and area per lipid increase beside lowering the pore nucleation free energy. Model polyarginine R8 similarly promoted pore formation in simulations, but without overall bilayer destabilization. We conclude that while the guanidine moiety is intrinsically membrane-disruptive, poly-arginines favor pore formation in negatively charged membranes via a different mechanism. Pore formation by R-rich peptides seems to be counteracted by lipids with PC headgroups. We found that long $R_n$ and $R_{Side}$ but not short $R_n$ reduce the free energy of nucleating a pore. In short $R_n$ , the substantial effect of the charged termini prevent their membrane activity, rationalizing why only longer $R_n$ are membrane-active.
