Cooperative Effects of an Antifungal Moiety and DMSO on Pore Formation over Lipid Membranes Revealed by Free Energy Calculations
Abstract
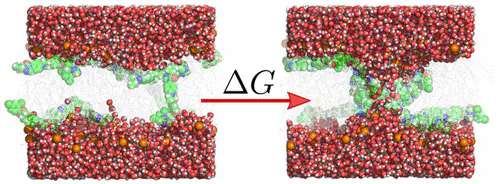
Itraconazole is a triazole drug widely used in the treatment of fungal infections, and it is in clinical trials for treatment of several cancers. However, the drug suffers from poor solubility, while experiments have shown that itraconazole delivery in liposome nanocarriers improves both circulation half-life and tissue distribution. The drug release mechanism from the nanocarrier is still unknown, and it depends on several factors including membrane stability against defect formation. In this work, we used molecular dynamics simulations and potential of mean force (PMF) calculations to quantify the influence of itraconazole on pore formation over lipid membranes, and we compared the effect by itraconazole with a pore-stabilizing effect by the organic solvent dimethyl sulfoxide (DMSO). According to the PMFs, both itraconazole and DMSO greatly reduce the free energy of pore formation, by up to ∼20 kJ mol–1. However, whereas large concentrations of itraconazole of 8 mol % (relative to lipid) were required, only small concentrations of a few mole % DMSO (relative to water) were sufficient to stabilize pores. In addition, itraconazole and DMSO facilitate pore formation by different mechanisms. Whereas itraconazole predominantly aids the formation of a partial defect with a locally thinned membrane, DMSO mainly stabilizes a transmembrane water needle by shielding it from the hydrophobic core. Notably, the two distinct mechanisms act cooperatively upon adding both itraconazole and DMSO to the membrane, as revealed by an additional reduction of the pore free energy. Overall, our simulations reveal molecular mechanisms and free energies of membrane pore formation by small molecules. We suggest that the stabilization of a locally thinned membrane as well as the shielding of a transmembrane water needle from the hydrophobic membrane core may be a general mechanism by which amphiphilic molecules facilitate pore formation over lipid membranes at sufficient concentrations.