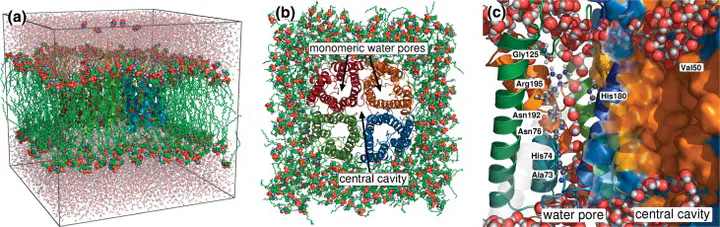
Abstract
Aquaporins facilitate water permeation across biological membranes. Additionally, glycerol and other small neutral solutes are permeated by related aquaglyceroporins. The role of aquaporins in gas permeation has been a long-standing and controversially discussed issue. We present an extensive set of atomistic molecular dynamics simulations that address the question of CO2 permeation through human aquaporin-1. Free energy profiles derived from the simulations display a barrier of ∼23kJ/mol in the aromatic/arginine constriction region of the water pore, whereas a barrier of ∼4kJ/mol was observed for a palmitoyloleoylphosphatidylethanolamine lipid bilayer membrane. The results indicate that significant aquaporin-1-mediated CO2 permeation is to be expected only in membranes with a low intrinsic CO2 permeability.