CO$_2$ and O$_2$ Distribution in Rubisco Suggests the Small Subunit Functions as a CO$_2$ Reservoir
Abstract
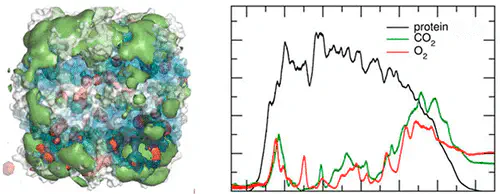
Protein–gas interactions are important in biology. The enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) catalyzes two competing reactions involving CO$_2$ and O$_2$ as substrates. Carboxylation of the common substrate ribulose-1,5-bisphosphate leads to photosynthetic carbon assimilation, while the oxygenation reaction competes with carboxylation and reduces photosynthetic productivity. The migration of the two gases in and around Rubisco was investigated using molecular dynamics simulations. The results indicate that at equal concentrations of the gases, Rubisco binds CO$_2$ stronger than it does O$_2$. Amino acids with small hydrophobic side chains are the most proficient in attracting CO$_2$, indicating a significant contribution of the hydrophobic effect in the interaction. On average, residues in the small subunit bind approximately twice as much CO$_2$ as do residues in the large subunit. We did not detect any cavities that would provide a route to the active site for the gases. Instead, CO$_2$ appears to be guided toward the active site through a CO$_2$ binding region around the active site opening that extends to the closest neighboring small subunits. Taken together, these results suggest the small subunit may function as a “reservoir” for CO$_2$ storage.